 眼下,在东北黑土地“国家大粮仓”的稻田里,一望无际的
【阅读全文】
眼下,在东北黑土地“国家大粮仓”的稻田里,一望无际的
【阅读全文】
 7月29日,中共中央政治局常委、国务院总理李克强到国家
【阅读全文】
7月29日,中共中央政治局常委、国务院总理李克强到国家
【阅读全文】
 2016年被称为不动产统一登记制度“落地”之年。年已过
【阅读全文】
2016年被称为不动产统一登记制度“落地”之年。年已过
【阅读全文】
时间:2016-08-03 来源:转基因观察
作者:Claire Robinson;翻译:jrry86;
时间:2016年7月20日;
原文链接:http://www.gmwatch.org/news/latest-news/17121-emails-reveal-role-of-monsanto-in-seralini-study-retraction
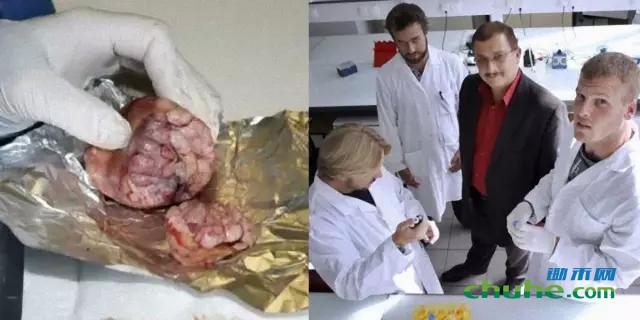
《食品和化学毒理学》杂志的主编邀请孟山都的科学家参与评审发现孟山都产品毒性的研究,法国《世界报》报道
2012年9月《食品与化学毒理学》杂志(FCT)发表了法国生物学家塞拉利尼教授团队的研究,他们发现用孟山都转基因玉米NK603和很小剂量的农达除草剂(种植该玉米时使用的除草剂)长期喂养大鼠,会导致肝肾毒性和内分泌紊乱。他们还观察到大多数试验组的大鼠表现出肿瘤增加趋势。
2013年11月,在将一个前孟山都科学家Richard E. Goodman安排进杂志编委会,并经过一个长达数月的由无名人士参与的不透明的评审程序之后,杂志主编A. Wallace Hayes将该文章撤回。
孟山都是否有向《食品和化学毒理学》杂志(FCT)施加压力以撤回文章?法国记者Stéphane Foucart在《世界报》的一篇文章(http://www.lemonde.fr/sciences/article/2016/07/11/la-discrete-influence-de-monsanto_4967784_1650684.html?xtmc=goodman&xtcr=1)中探讨了这个问题。
文章揭示Goodman完全附属于孟山都。它还揭露了Hayes是如何在塞文的撤回事件中发挥双重作用的,他在幕后操作以鼓励孟山都科学家加入评审小组,并用他们的观点来影响撤回的决定。
化学公司对学者的影响
美国食品透明组织“美国有权知道”(USRTK)根据信息自由法获取并公开了这一事件中的相关电子邮件,Foucart检视了这些邮件。他写道,这些电邮“揭示了化学公司对一些学者的影响”。
Foucart指出科学文章被撤回的原因通常是造假、抄袭或诚信问题。塞拉利尼的研究与这些完全无关,它是第一个因为结论“不确定”而被撤回的。塞拉利尼的支持者把矛头指向了FCT编委会负责生物技术口的新人。Richard E. Goodman是位于美国林肯市的内布拉斯加大学的教授,食品过敏研究专家。他也是孟山都前雇员,于2004年离开。
FCT生物技术编辑与孟山都有“非同寻常的关系”
Foucart写道,USRTK获得的电邮显示Goodman与他的老雇主之间有“非同寻常”的关系。实际上,正如Foucart指出的那样,Goodman与孟山都之间的关系并不像看上去的那样是过去式。Goodman自己在2012年写到“他工资的一半”来自由孟山都、拜耳、巴斯夫、陶氏、杜邦和先正达资助的一个项目,这个项目是建立一个食品过敏源数据库。
Foucart指出这一事实使Goodman与孟山都产生“关联,甚至是从属关系”。Foucart进一步说明了这样的从属关系是如何在2012年5月发生的一个插曲中表现出来的(在塞拉利尼文章9月份发表之前):
“一份报纸发表的一篇文章引用了Goodman的一个观点,那时他还不是FCT编辑。这个观点导致一个孟山都雇员对他上纲上线。该雇员对Goodman教授说,他的观点似乎被记者解释成'暗示我们对生物技术了解不够,所以不能保证其安全'。于是Goodman给上述资助他研究工作的所有六个生物公司的联系人集体发了回信。他说'我向你及贵公司道歉',他还说那个记者误解了他的意思。”
工业界通过资助科学而控制了研究者
Foucart对此评论道,来自工业界对科学研究的资助,对大学研究人员来说不仅意味着简单的知识产出,而是远比这更大的责任:“它对研究人员的公开言论施加了某种形式的控制。”2012年8月,Goodman作了个榜样,他提醒他的资助者们说国家公共电台将就转基因安全性采访他。他的一个联系人问他:“你是不是愿意在采访前参加一个如何与媒体打交道的培训?”不清楚Goodman后来是否接受了这个建议,因为他没有回应《世界报》的询问。
一个月后,即2012年9月,塞拉利尼的研究得以发表,那时Goodman仍然还不是FCT编委会成员。Foucart写道,9月19日Goodman向他在孟山都的联系人通报了塞拉利尼文章发表的消息,并说如果公司能给他提供一些批判材料,他将“不甚感激”。孟山都联系人回应道:“我们正在评估这篇文章,等我们有了论据就会告诉你。”Foucart继续写道,几天后Goodman被时任FCT总编的毒理学家Wallace Hayes任命为FCT的副总编。
这个认命一直到2013年2月都没有公开宣布。Foucart指出,Goodman加入编委会是塞拉利尼文章发表的直接和即时的结果。2012年11月2日,“塞拉利尼事件”完全发酵,Hayes在给孟山都雇员的电邮中宣布从现在开始Goodman将掌管杂志的生物技术口。Hayes补充说:“我作为主编,与Goodman教授一道请求你们中对最近塞拉利尼等人的文章持强烈批评态度的人,主动要求成为可能的评审员人选。”
Foucart评论说,Hayes正式邀请孟山都毒理学家参与评估提交给杂志供评审的那些转基因研究,以决定是接受还是拒绝。《世界报》参考的电邮文件中并没有说Hayes是否仅仅邀请了孟山都科学家,而Hayes也没有对《世界报》的询问作出回应。
这些说明我们“转基因观察”网对Goodman加入编委会的质疑(https://www.independentsciencenews.org/science-media/the-goodman-affair-monsanto-targets-the-heart-of-science/)是正确的。这还同时说明我们对第二轮不透明的评审的批评是正确的(第一轮评审指的是同意塞拉利尼论文发表的同行评议)。一些观察家对我们说,他们认为评审人员匿名是可以接受的,因为同行评议通常都是一个匿名过程。但是这个针对已经通过同行评议的、发表了已经一年的文章所作的特定的'评审',从一开始就采用了一个高度暧昧而不合规范的处理方式,特别是这篇文章除了最后没有得出确定无疑的结论(无数科学文章都是如此)之外,其本身没有任何错误。因此我们相信评审员的身份和利益关系应该公开。
另一个批评转基因的研究也被FCT拒绝
Foucart指出没有办法确知所有这些是否对该杂志会接受什么样的文章造成影响。但是根据他获得的信息,2013年,FCT还拒绝了发表用孟山都转基因玉米MON810对大型水蚤(一种水蚤)所作的学术界第一个长期毒性研究。这个研究表明对这种小型淡水甲壳纲动物有毒害作用,而它是生态毒理学家用来作为研究模型的生物。
是Goodman通知作者拒稿决定的,他强调是因为评审者的负面评价。最后这项研究于2015年发表在另一个杂志上。与塞拉利尼的研究不太一样的是,这项研究没有受到挑战(可能是因为不像大鼠喂养试验,该试验不会被管理部门认为与人类相关)。
Goodman为反驳(对转基因的)批评而向孟山都寻求科学论据
Foucart报告说,在某些场合,Goodman似乎不得不听从孟山都毒理学家的判断,因为他评审的一篇文章有些内容超出了他的知识范围。他在2014年10月给他的一个孟山都联系人写信说:“我正在评审一个'反'(推测是'反转基因或农药')文,他们引用了一个2014年斯里兰卡的研究,后者指出接触草甘膦与肾病之间可能有关联,还提出了一个机理(来解释这种毒性作用)”。他补充说:“我不是好的化学家或毒理学家,不太明白他们的逻辑的强弱:你能给我一些关于其逻辑是否说的通的可靠的科学论据么?”
草甘膦是农达除草剂的活性成分,而农达是孟山都的重要产品,与该公司的转基因抗草甘膦作物捆绑销售。
根据Foucart的说法,《世界报》参考的文件中,没有证据支持Goodman在塞拉利尼研究被撤回这一事件中起到作用---那项决定是由Hayes作出的。2015年1月,Goodman以时间有限为由向杂志辞职。
Hayes的利益冲突
但是很明显Hayes在撤回事件中起到了关键作用。他牵涉到很多利益冲突,可能影响他的决策。
在Hayes的职业生涯中他长期担任工业界的毒理学家。他现在是Spherix咨询公司的高级科学顾问(http://bit.ly/2arexiW),”该公司集中了全球富有经验的顾问团队,为来自食品、食品补剂、消费产品和制药工业的客户提供科学解决方法以帮助他们成功管理。”
Hayes以前的职务包括(http://bit.ly/2arexiW):
* 食品巨头RJR Nabisco的副总裁(译注:这是美国公司的一种职称、头衔,而非职务)和公司毒理学家,负责处理世界范围内的与食品和饮料产品中的相关成分和食品接触物质的安全性有关的所有管理以及毒理学方面的问题(译注:食品接触物质是指食品生产、包装、运输等过程中会接触到的物质)。
* 吉列公司的产品信誉部门的公司副总裁,负责“安全评估,以确保各种消费产品、工厂安全、环境管理和质量控制等等符合管理规定。在吉列期间,他负责全球范围的涉及管理和毒理学方面的问题。所有用在吉列产品(包括个人护理产品)中的接触物质都要在他的部门获得批准。
工业利益凌驾于科学之上
因为Hayes的利益冲突以及Goodman与孟山都的关系,他们应该被剥夺决定塞拉利尼研究和提交给FCT的其它研究的命运的权力。而且向Hayes提供意见导致他决定撤回塞氏研究的那些评审者的名字应该公开,任何有利益冲突的人都应被排除出评审小组。
可实际情况却是FCT的程序不透明使得工业界的利益凌驾于科学考量之上。在这个过程中,诚实科学家的名誉受到不公正的诽谤,公众对科学的信任受到伤害,这也许是不可挽回的。
以下是英文原文:
Emails reveal role of Monsanto in Séralini study retraction
Editor at the journal Food and Chemical Toxicology invited Monsanto scientists to review a study that found toxic effects from Monsanto products, reports French newspaper Le Monde
In September 2012 the journal Food and Chemical Toxicology (FCT) published the research of a team led by the French biologist Professor Gilles-Eric Séralini, which found liver and kidney toxicity and hormonal disturbances in rats fed Monsanto’s GM maize NK603 and very small doses of the Roundup herbicide it is grown with, over a long-term period. An additional observation was a trend of increased tumours in most treatment groups.
In November 2013 the study was retracted by the journal’s editor, A. Wallace Hayes, after the appointment of a former Monsanto scientist, Richard E. Goodman, to the editorial board and a non-transparent review process by nameless people that took several months.
Did Monsanto pressure the journal Food and Chemical Toxicology (FCT) to retract the study? French journalist Stéphane Foucart addresses this question in an article for Le Monde.
The article shows the total subordination of Goodman to Monsanto. It also reveals how Hayes played a double role in the retraction of the study, acting behind the scenes to encourage Monsanto scientists to join the reviewing panel that would feed their views into the decision to retract.
Foucart examined emails disclosed as a result of a freedom of information request submitted by the food transparency organisation US Right to Know (USRTK). Foucart writes that the emails “reveal the influence of the chemical companies on some academics”.
Foucart points out that scientific papers are normally retracted only due to fraud, plagiarism, or honest error. Séralini’s study did not fall into any of these categories and was the first to be retracted on grounds of "inconclusiveness”. Supporters of Séralini challenged a newcomer to the editorial board of FCT, in charge of biotechnology. Richard E. Goodman is a Professor at the University of Nebraska in Lincoln (USA) and specialist food allergens. He is also a former employee of Monsanto, which he left in 2004.
Foucart writes that emails obtained by USRTK show “a remarkable closeness” between Goodman and his old employer. In reality, however, as Foucart points out, the relationship between Goodman and Monsanto is not so old. Goodman himself wrote in a message of 2012 that "50% of [his] salary” actually comes from a project funded by Monsanto, Bayer, BASF, Dow, DuPont and Syngenta, and consists of establishing a database of food allergens.
This fact, Foucart notes, creates “links, or even a subordinate relationship” between Goodman and Monsanto. Foucart goes on to explain how that subordinate relationship manifested in an incident that happened in May 2012, before the publication of the Séralini paper in September of that year:
“After the publication of a newspaper article in which he is quoted, Goodman, not yet an editor at FCT, is sharply brought to order by a Monsanto employee. The latter tells the professor that his opinion seems to have been interpreted by the journalist as ‘suggesting that we do not know enough about biotechnologies to say that they are safe’. In return, Goodman wrote a collective message to all his correspondents in the six biotechnology companies that fund his work. ‘I apologize to you and your companies,’ he wrote, adding that he was misunderstood by the journalist.”
Foucart comments that the financing of scientific work by industry means for university researchers a commitment that goes far beyond the simple production of knowledge: “It imposes a form of control over the public discourse of the researcher.” In August 2012, Goodman took the lead and warned his sponsors that he would be interviewed by National Public Radio on the safety of GMOs. "Would you participate in a media training session before the interview?”, asked one of his correspondents. It is not known whether Goodman accepted this proposal, as he did not respond to Le Monde’s inquiries.
A month later, in September 2012, the study by Gilles-Eric Séralini was published. Goodman was not at the time a member of the editorial board of FCT. On 19 September, Foucart writes, Goodman informed his Monsanto correspondent about the publication of the Séralini’s article and that he “would appreciate" it if the firm could provide him with criticisms. "We're reviewing the paper,” the Monsanto correspondent replies. “I will send you the arguments that we have developed." A few days later, Foucart writes, Goodman was named “associate editor" of FCT, on the decision of the toxicologist Wallace Hayes, then editor of the journal.
This appointment was not publicly announced until February 2013. Foucart notes that the addition of Goodman on the editorial board of the magazine was actually a direct and immediate consequence of the publication of Seralini. On November 2, 2012, when the "Séralini affair” was in full flow, Hayes announced in an email to Monsanto employees that Goodman would from now on be in charge of biotechnology at the journal. Hayes added: "My request, as editor, and from Professor Goodman, is that those of you who are highly critical of the recent paper by Séralini and his co-authors volunteer as potential reviewers."
Foucart comments that Hayes was formally inviting Monsanto toxicologists to appraise for acceptance or rejection studies on GMOs that are submitted to the journal for review. The documents consulted by Le Monde did not say if Hayes - who has not responded to Le Monde’s inquiries - limited this request to Monsanto scientists.
This confirms that we at GMWatch were right to question the arrival of Goodman on the editorial board. It also shows that we were right to criticise the non-transparency of the second round of review (the first review being the one that led to the study being published). Some observers told us they thought it was acceptable that the reviewers remained anonymous, since peer-review is generally an anonymous process. But this particular ‘review’ – of a paper that had already passed peer-review, had been published for a year, and had nothing wrong with it beyond the fact that, in common with countless other scientific papers, it was “inconclusive” on some endpoints –
Foucart notes that there is no way of knowing for sure if all this had an impact on articles accepted by the journal. But in 2013, according to information received by him, FCT rejected the first academic study of chronic toxicity of a Monsanto GM maize - MON810 - in Daphnia magna, a type of waterflea. The study suggested harmful effects on this small freshwater crustacean, which is used as a model organism by ecotoxicologists.
It was Goodman who informed the authors of the rejection, highlighting the negative reports of the peer reviewers. The study was eventually published in 2015 in another journal. Unlike the Séralini study, it was not challenged (probably because, unlike a rat feeding study, such a study would not be deemed by regulators to be relevant to humans).
In some cases, Foucart reports, Goodman seems to defer to the judgement of Monsanto's toxicologists which he has to evaluate an article containing aspects that are beyond his knowledge. "I'm looking at an ‘anti’ [presumably ‘anti-GMOs or pesticides’] paper,” he wrote in October 2014 to one of his Monsanto correspondents. “They cite a Sri Lankan study of 2014 on a possible link between glyphosate exposure and kidney disease, as well as a mechanism [to explain this toxicity]." Goodman added: "I'm not enough of a chemist or toxicologist to understand the strengths and the weaknesses of their logic: can you give me some solid scientific arguments about whether it is, or is not, plausible."
Glyphosate, the active ingredient of Roundup herbicide, is a key product of Monsanto, as it is sold with the company’s GM glyphosate-tolerant crops.
According to Foucart, nothing in the documents consulted by Le Monde supports the idea that Goodman played a role in the retraction of the Séralini study - that decision was taken by Hayes. In January 2015, Goodman resigned his position at the journal, due to time constraints.
However, Hayes clearly did play a key role in the retraction. And he has plenty of conflicts of interest that might have influenced his decision.
Hayes has had a long career as an industry toxicologist. He is senior science advisor at Spherix Consulting, “a global team of experienced advisors who provide their clients in the food, dietary supplement, consumer product, and pharmaceutical industries with scientific solutions that result in regulatory success.”
Hayes's previous appointments include:
* Vice President and Corporate Toxicologist for food giant RJR Nabisco, with responsibility for all regulatory and toxicology issues related to the safety of ingredients and food contact substances for food and drink products worldwide.
* Corporate Vice-President of Product Integrity at the Gillette Company, with responsibility for “the safety evaluation and regulatory compliance of a variety of consumer products, plant safety, environmental stewardship, and quality control. While at Gillette, Dr Hayes was responsible for managing regulatory and toxicology issues worldwide. All contact substances used in Gillette products (including personal care products) were cleared within his division.”
Hayes’s interests and Goodman’s current Monsanto connections should have precluded them from having any authority over the fate of the Séralini study and other studies submitted to FCT. In addition, the names of the reviewers who fed their views into Hayes’s decision to retract the study should have been declared and made public and any conflicts of interest should have led to exclusion from the panel.
Instead we have a situation in which a lack of transparency at the journal FCT allowed industry interests to take precedence over scientific considerations. In the process, the reputation of honest scientists has been unjustly maligned and public trust in science has been damaged, perhaps irretrievably.
Report: Claire Robinson
更多猛料!欢迎扫描下方二维码关注锄禾网官方微信(chuhewang)。

